Publications
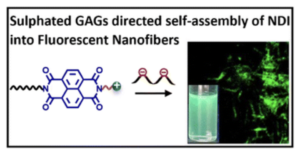
Selective Binding of Sulphated Glycosaminoglycans Induces Self-assembly of Naphthalene Diimide into Fluorescent Nanofibers
P. Sharma, E. Fernández-Pedrera Lópeza, B. Cantero Nieto, A. Calò, S. Ghosh, P. Rodríguez, X. Companyó, B. Limburg and Mohit Kumar*
Nanoscale 2026 | DOI: 10.1039/D5NR04833H

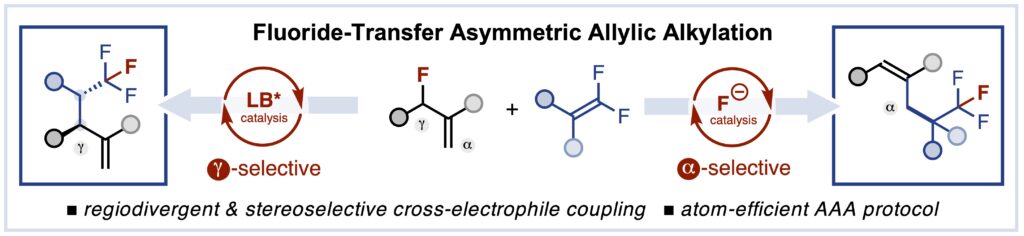
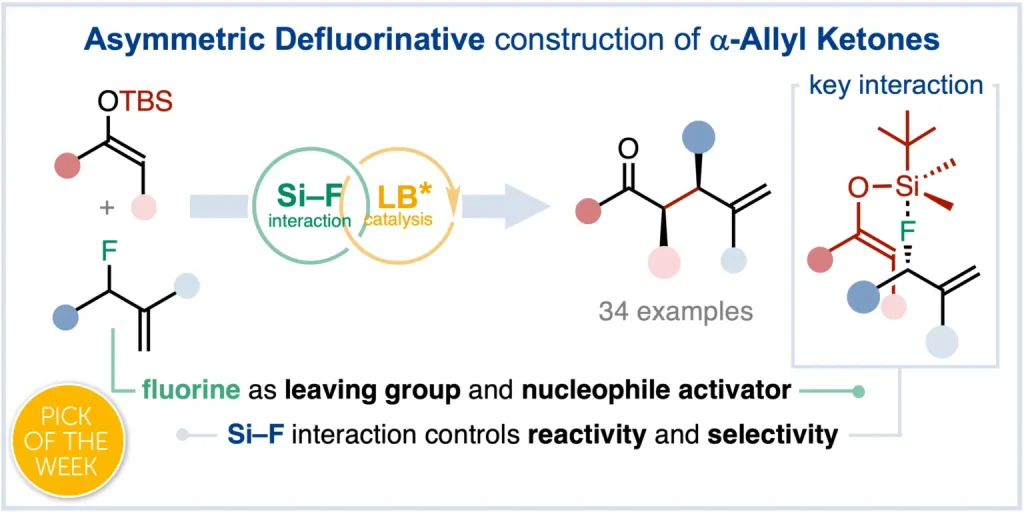
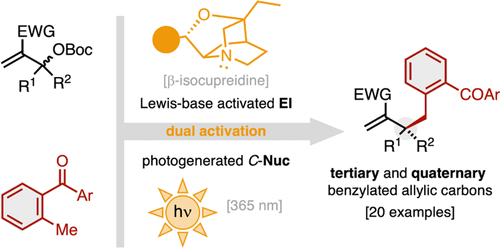
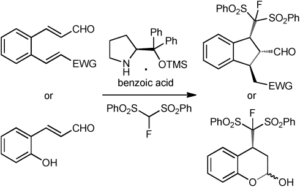
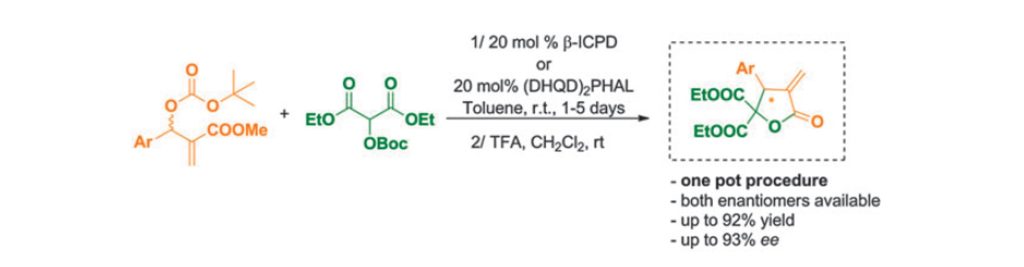
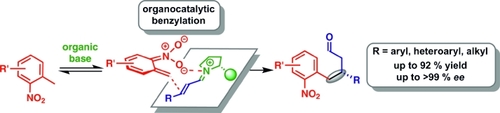
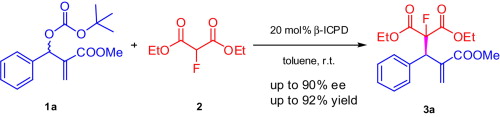
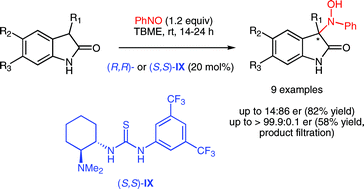
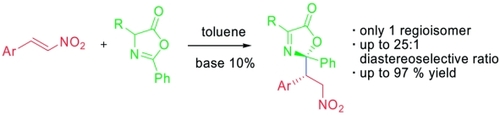
Fluoride-Transfer Asymmetric Allylic Alkylation Enables Regiodivergent, Stereoselective Cross-Electrophile Coupling
J. Duran, V. G. Moldoveanu, C. Barroso, G. A. Pereira, B. Limburg and X. Companyó*
Angew. Chem. Int. Ed. 2026, 65, e20513 | DOI: 10.1002/anie.202520513

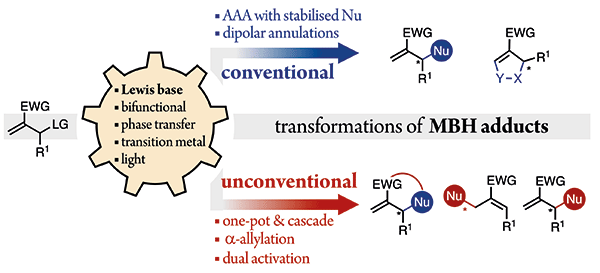
Chapter 26: Asymmetric Organocatalytic Conjugate Additions
A. Vega-Peñaloza, A. Moyano and X. Companyó*
Enantioselective Organocatalysis: Catalysts, Reactions, and Applications. Wiley-VCH Verlag GmbH & Co. 2025, Volume 2, part III, 971-1023 | DOI: 10.1002/9783527845552.ch26

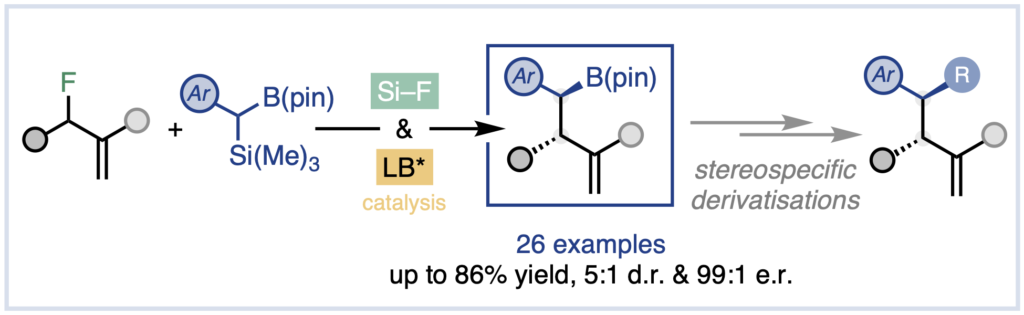
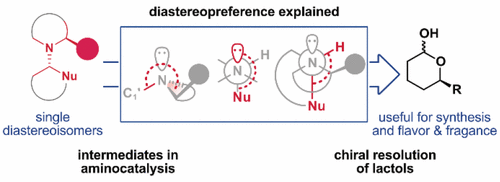
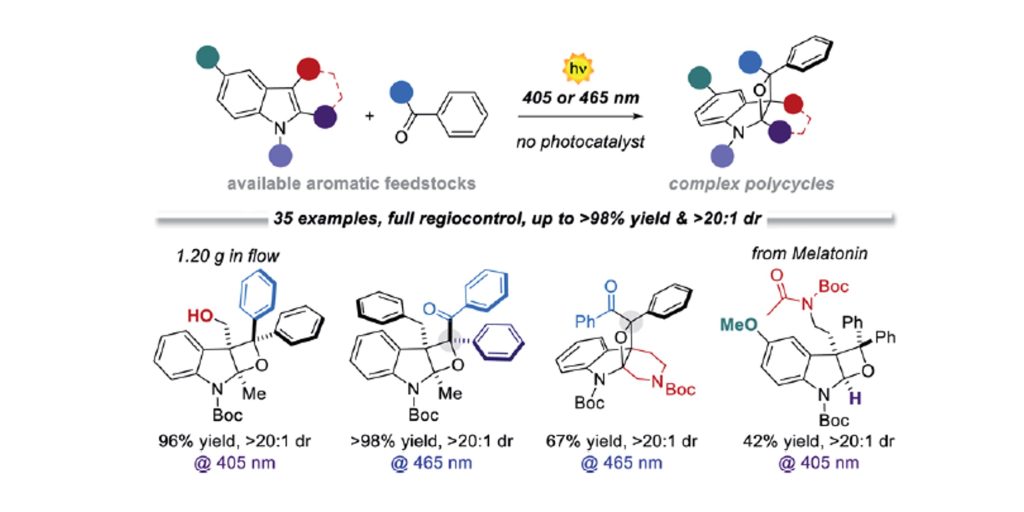
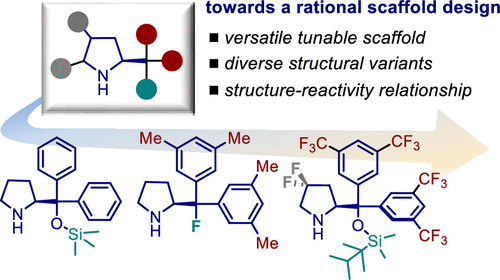
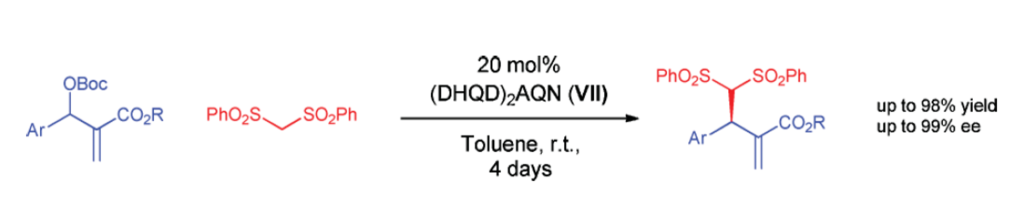
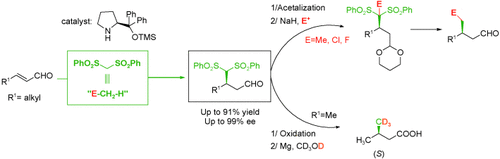
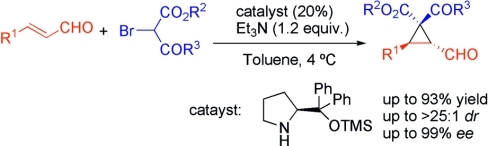
Org. Lett. 2024, 26, 8394-8399 | DOI: 10.1021/acs.orglett.4c03242 – highlighted in Organic Synthesis Newsletter | For a preliminary version deposited in ChemRxiv see DOI: 10.26434/chemrxiv-2024-7ld1j

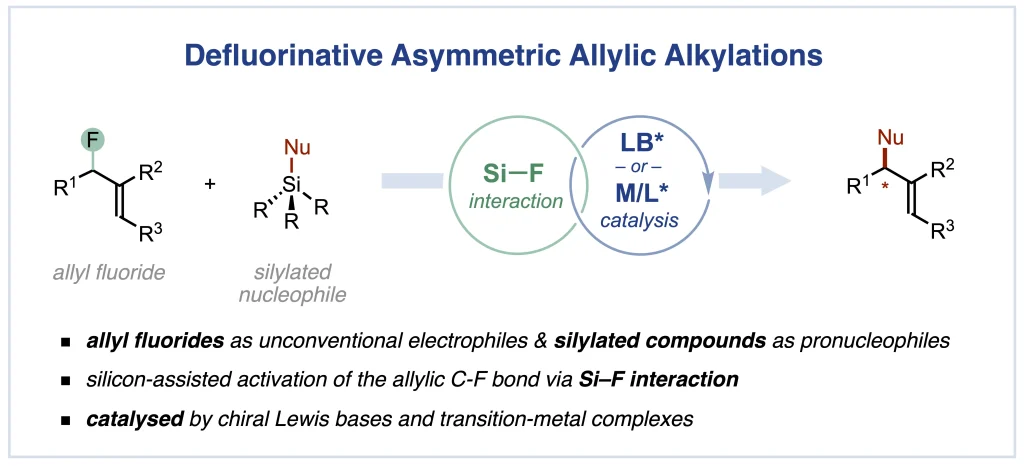
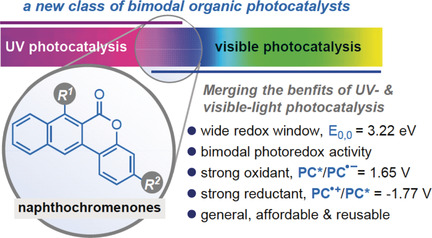
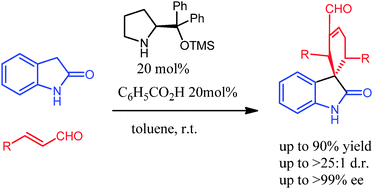
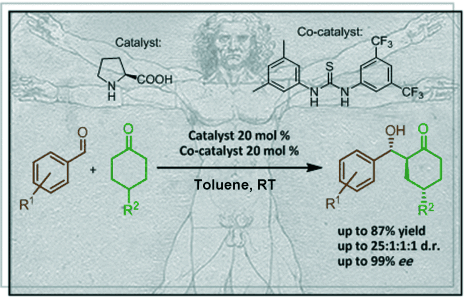
Synlett 2024, 35, 1613-1620 | DOI: 10.1055/a-2211-6538 – invited contribution to the 13th EuCheMS Young Investigators Workshop special issue

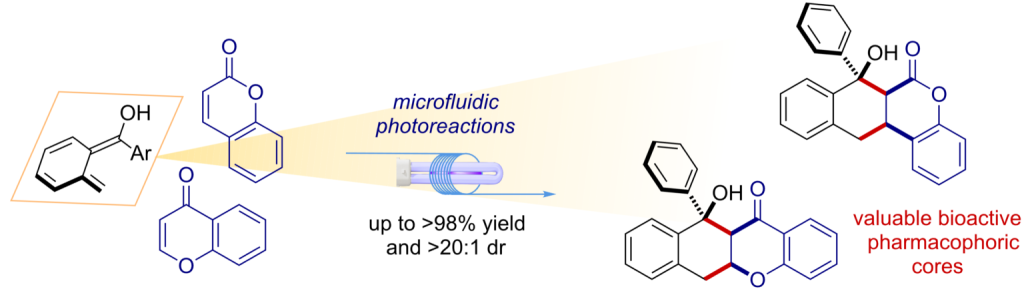
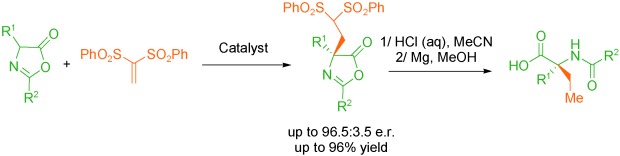
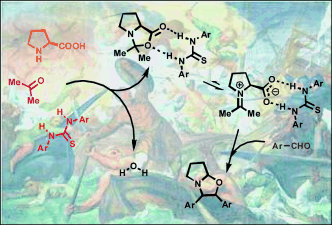
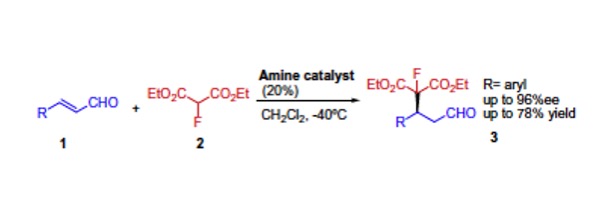
Chem. Sci. 2023, 14, 7147-7153 | DOI: 10.1039/D3SC01498C – paper selected as ChemSci Pick of the Week and HOT article | For a preprint version in ChemRxiv see DOI: 10.26434/chemrxiv-2023-wxmzv

Spiro Compounds: Synthesis and Applications; John Wiley & Sons, Inc. 2022, 35-64 | DOI: 10.1002/9781119567646.ch3; ISBN: 9781119567646

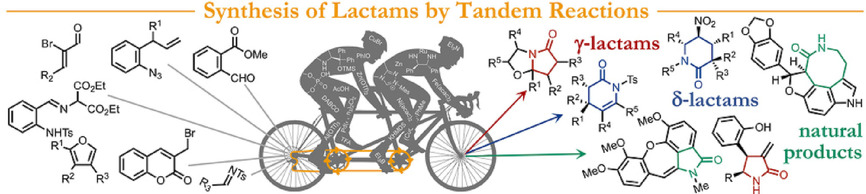
J. Org. Chem. 2021, 86, 4326-4335 | DOI: 10.1021/acs.joc.0c02998

Angew. Chem. Int. Ed. 2021, 60, 1082-1097 | DOI: 10.1002/anie.202006416

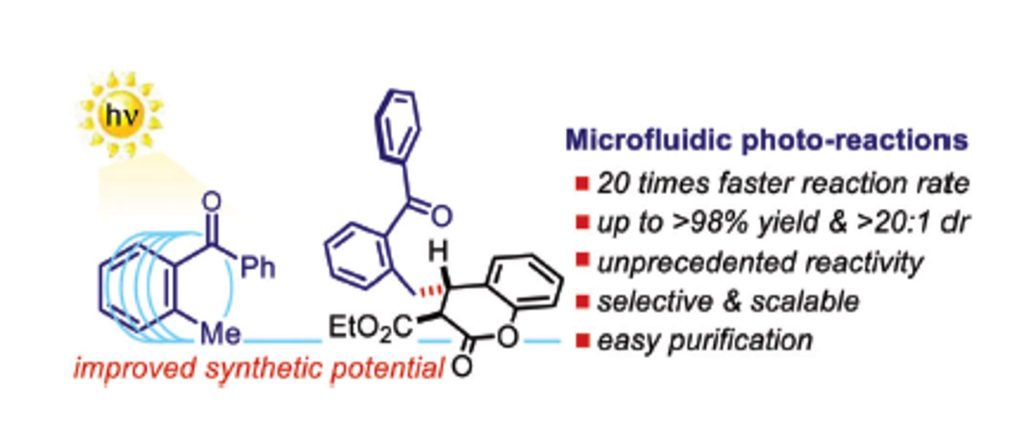
Chem 2020, 6, 3022-3037 | DOI: 10.1016/j.chempr.2020.08.009
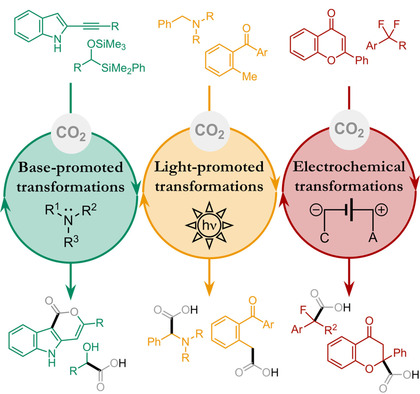
CO2 as building block in organic synthesis; Wiley-VCH Verlag GmbH & Co. 2020, 225-252 | DOI: 10.1002/9783527821952.ch7 ; ISBN: 9783527821952

J. Org. Chem. 2020, 85, 4463-4474 | DOI: 10.1021/acs.joc.0c00175

Synthesis 2020, 52, 2922-2932. DOI: 10.1055/s-0040-1707207 – Invited contribution Ed: Prof. P. Knochel–

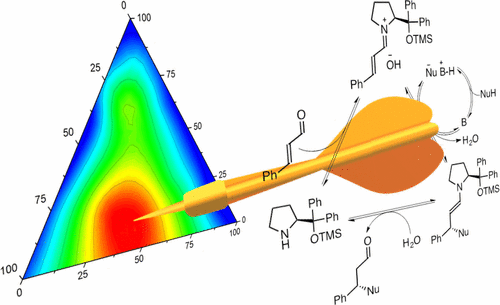
Chem. Sci. 2020, 11, 6532-6538 | DOI: 10.1039/D0SC01569E – for a preprint version in ChemRxiv see DOI: 10.26434/chemrxiv.11743752.v1

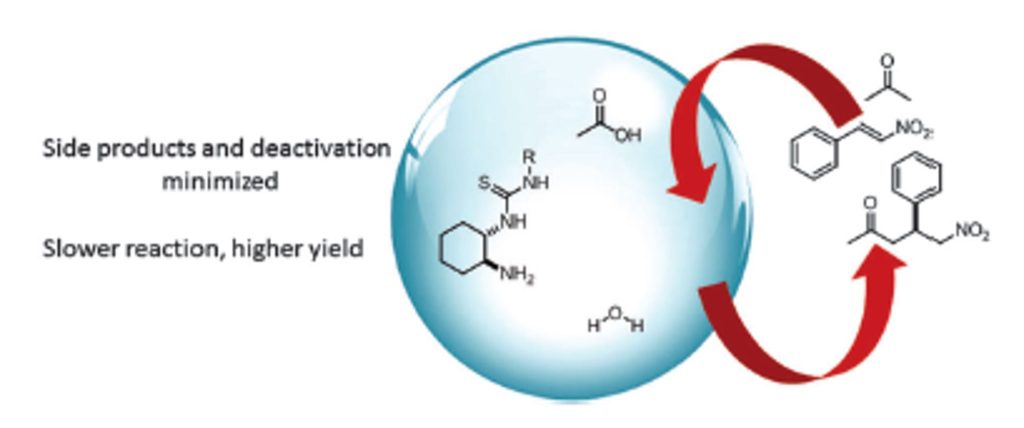
Angew. Chem. Int. Ed. 2020, 59, 1302-1312 | DOI: 10.1002/anie.201912455

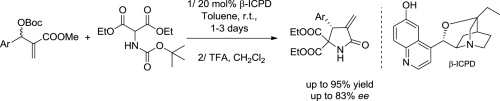
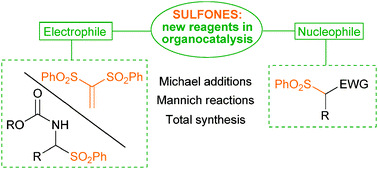
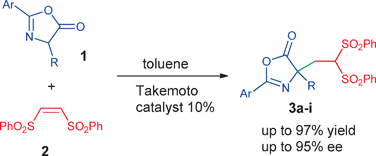
ACS Catalysis 2019, 9, 6058-6072 | DOI: 10.1021/acscatal.9b01556
Beilstein J. Org. Chem. 2018, 14, 2418-2424 | DOI: 10.3762/bjoc.14.219 – invited contribution to “Photoredox catalysis for novel organic reactions” thematic issue

Asian J. Org. Chem. 2018, 7, 1934-1956 | DOI: 10.1002/ajoc.201800300

Chem. Commun. 2018, 54, 6820-6823 | DOI: 10.1039/C8CC01373J

ChemSusChem 2018, 11, 3056-3070 | DOI: 10.1002/cssc.201801063 – amongst the top 10% most downloaded ChemSusChem articles in 2018-2019 –
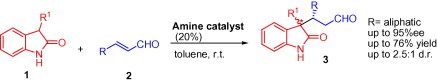
J. Am. Chem. Soc. 2017, 139, 8432-8435 | DOI: 10.1021/jacs.7b05045 – featured in the 2018 JACS Young Investigator Virtual Issue –

Chem. Commun. 2016, 52, 6821-6824 | DOI: 10.1039/C6CC01026A

Tetrahedron 2014, 70, 75-83 | DOI: 10.1016/j.tet.2013.11.028

Adv. Synth. Catal. 2014, 356, 437-446 | DOI: 10.1002/adsc.201300703

Comprehensive enantioselective organocatalysis: catalysts, reactions and applications; Wiley-VCH Verlag GmbH & Co. 2013, Volume 3, part III, 977-1012 | ISBN: 978-3-527-33236-6

Chem. Commun. 2013, 1184-1186 | DOI: 10.1039/C2CC38659C – highlighted in Synfacts 2013, 9, 0355 | DOI: 10.1055/s-0032-1318212 –

Stereoselective organocatalysis: bond formation methodologies and activation modes; Wiley 2013, 351-380 | ISBN: 978-1-118-60470-0

Stereoselective organocatalysis: bond formation methodologies and activation modes; Wiley 2013, 1-10 | ISBN: 978-1-118-60470-0

Eur. J. Org. Chem. 2013, 5262-5265 | DOI: 10.1002/ejoc.201300899

Studies in Natural Products Chemistry (Bioactive Natural Products); Elsevier Science Publishers 2013, 71-132 | ISBN: 978-0-444-596031

Tetrahedron Lett. 2012, 53, 4124-412 | DOI: 10.1016/j.tetlet.2012.05.121

X. Companyó, G. Valero, O. Pineda, T. Calvet, M. Font-Bardía, A. Moyano and R. Rios
Org Biomol. Chem. 2012, 10, 431-439 | DOI: 10.1039/C1OB06503C

Chem. Eur. J. 2011, 17, 2018-2037 | DOI: 10.1002/chem.201001546 – amongst the top 20 most cited papers in Chemistry-A European Journal in 20th anniversary of the journal (1995-2015) –


Org. Biomol. Chem. 2011, 9, 7986-7989 | DOI: 10.1039/C1OB06308A

Chem. Commun. 2010, 46, 6953-6955 | DOI: 10.1039/c0cc01522a

A.-N. Alba, X. Companyó, G. Valero, A. Moyano and R. Rios
Chem. Eur. J. 2010, 16, 5354-5361 | DOI: 10.1002/chem.200903025

Chem. Eur. J. 2010, 16, 1142-1148 | DOI: 10.1002/chem.200902678

A.-N. Alba, X. Companyó and R. Rios
Chem. Soc. Rev. 2010, 39, 2018-2033 | DOI: 10.1039/B911852G

New. J. Chem. 2010, 34, 1816-1820 | DOI: 10.1039/C0NJ00321B

Synlett 2010, 13, 1833-1908 | DOI: 10.1055/s-0030-1257988

Mini Rev Org Chem 2010, 7, 1-9 | DOI: 10.2174/1570193X11007010001

Tetrahedron Lett. 2009, 50, 6624-6626 | DOI: 10.1016/j.tetlet.2009.09.038

Chem. Eur. J. 2009, 15, 11095-11099 | DOI: 10.1002/chem.200901806

Tetrahedron Lett. 2009, 50, 5021-5024 | DOI: 10.1016/j.tetlet.2009.06.092

Chem. Eur. J. 2009, 15, 7035-7038 | DOI: 10.1002/chem.200900991

Chem. Eur. J. 2009, 15, 6564-6568 | DOI: 10.1002/chem.200900488

Targets in heterocyclic chemistry; Springer 2009, 13, 147-174. Chapter 5 | ISBN: 978-88-86208-62-8

Cur Org Chem 2009, 13, 1432-1474 | DOI: 10.2174/138527209789055054

Eur. J. Org. Chem. 2009, 3075-3080 | DOI: 10.1002/ejoc.200900209

Lett. Org. Chem. 2009, 6, 293-296 | DOI: 10.2174/157017809788489855

A.-N. Balaguer, X. Companyó, T. Calvet, M. Font-Bardía, A. Moyano and R. Rios
Eur. J. Org. Chem. 2009, 199-203 | DOI: 10.1002/ejoc.200801005